I’m working on a pretty big life-philosophy-society type post right now, but I’ve been too busy to sit down and think, and too left-brained to generate effective rhetoric, so let’s talk about something a bit easier literary-wise. Anyone who knows me personally well enough that they know what I intend to major in my studies will know that I’m a pretty big science geek. And as a product of this, science and STEM have found a way to worm themselves into my projects from time to time. The most significant of these science-affected projects is my largest pet project, the Plastic Pyrolysis plant.
Those brave few with experience with the topic are likely foaming at the mouth right now, but I’ll give a short summary for those less chemically inclined. Plastic Pyrolysis functions on the principle that plastic is made out of oil, and as such can be made back into oil. However, anybody who’s ever lit plastic on fire before will know that it isn’t that simple. By introducing a closed environment and a lack of oxygen, plastic heated between 400 and 800 degrees Celsius will vaporize, instead of combust or melt. This “Plastic Vapor” is a rich mixture of various chain-length hydrocarbons, which can be condensed using a cooling system into energy-rich gases, and crude oil.
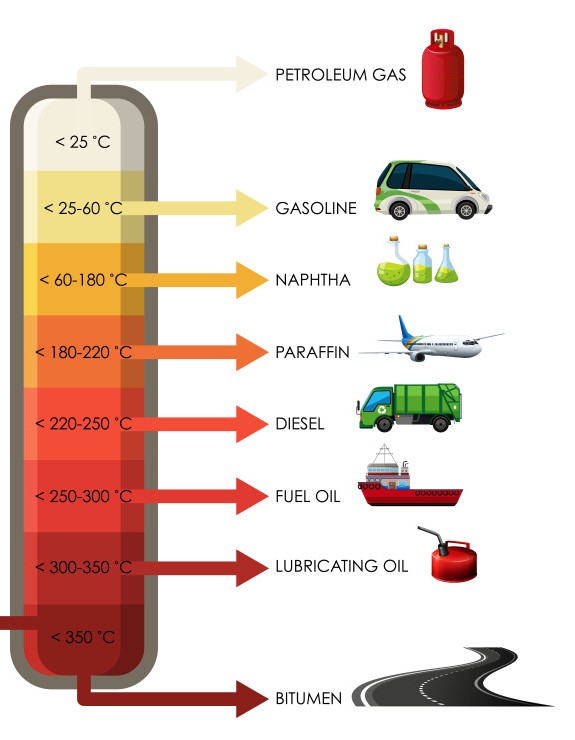
Practical applications of hydrocarbon fractions in crude oil obtained via pyrolysis
Why is this cool? This process turns discarded waste plastic into usable hydrocarbon-based chemicals. In simple terms, pyrolysis turns landfills into gasoline, diesel, propane, and all the other fossil fuels we wage wars with other countries to obtain. While objectively, it is not and never will be fully energy efficient, it is an amicable solution for reusing waste and a far better solution than anything currently available. And, even at the small scale I plan to apply it, it is still a fun and important proof of concept.

©2025 Ian Frost
So what’s my take on it? Pictured above is a detailed diagram of my preliminary design for a first-stage plastic pyrolysis plant. This design uses a carburated butane burner to heat an insulated vertical-outlet crucible, applying a Countercurrent Liebig Condenser to handle the condensation process, and a fume burner to expel unwanted gases. Using the gas-forming reaction of Hydrochloric Acid and Bicarbonate to produce the excess CO2 needed to flush the combustion chamber, this design is the greatest balance of cost efficacy and production efficiency possible. The double-walled insulation chamber is useful for minimizing heat loss, along with maintaining high temperatures enough to ignite all available fuel, maximizing fuel efficiency.
However, while this design is a cost-effective first-stage solution, it is far from the intended final design. Second-stage plans include utilizing a gas compressor to route the hydrocarbon-rich gas into the combustion burner, drastically improving energy utilization and minimizing energy loss. While it seems far-fetched, I believe with the right tools and funding at my disposal, this project is completely attainable, and plan to work towards its fruition in the near future.
Thanks for hearing out my odd dreams of crude oil production. And who knows, maybe this little project could be a push in the right direction for the future of sustainability amongst a trash-filled earth.